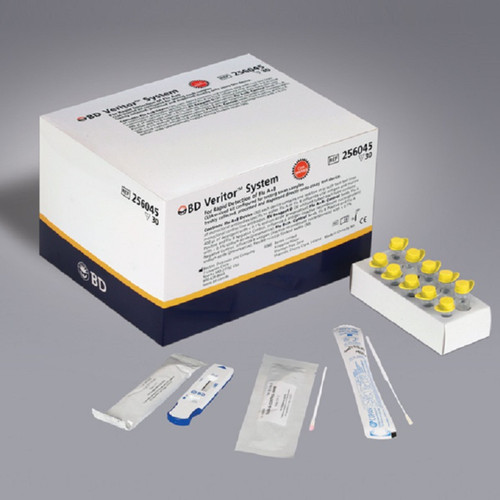
BD Veritor™ Plus System Influenza A+B Test Kit (30 Tests) – BD 256045
The BD 256045 is a professional-grade rapid diagnostic immunoassay designed for the qualitative detection of influenza A and B viral antigens. Optimized for use with the BD Veritor™ Plus Analyzer, this CLIA-waived kit provides digital, objective results in just 10–15 minutes, eliminating the subjectivity of visual interpretation.
Why Choose the BD Veritor™ Flu A+B Kit?
In clinical and urgent care settings, speed and accuracy are paramount. The
BD Veritor™ System utilizes "Adaptive Read Technology" to enhance sensitivity and reduce false-positive results. This 30-test box is the gold standard for point-of-care (POC) respiratory virus screening during peak flu seasons.
Key Features & Benefits
- Rapid, Digital Results: Get clear "Positive" or "Negative" readouts on the Veritor Plus Analyzer in as little as 10 minutes.
- CLIA-Waived Simplicity: Approved for use in non-traditional laboratory settings, including pharmacies, physician offices, and urgent care centers.
- Dual Detection Capability: Simultaneously detects and differentiates between Influenza A and Influenza B from a single nasal or nasopharyngeal swab.
- Advanced Workflow Efficiency: Features "Walk Away" and "Analyze Now" modes, allowing staff to multitask while samples incubate.
- Objective Reading: The analyzer corrects for non-specific binding and detects faint lines that the human eye might miss.
Kit Contents (Box of 30)
Each BD 256045 box comes fully equipped for 30 individual tests:
- 30 Individually pouched test devices
- 30 Tubes of RV Reagent D
- 30 Flexible minitip flocked swabs
- 1 Positive Control Swab (Flu A+/B-)
- 1 Negative Control Swab (Flu B+/A-)
Professional Specifications
- Manufacturer: BD (Becton Dickinson)
- Product Number: 256045
- Sample Type: Nasal or Nasopharyngeal Swab
- Storage Requirements: 2°C to 30°C (Do not freeze)
- Regulatory Status: CLIA-Waived for professional use